About us
SpineCraft fosters an environment of surgeon participation in the implant and instrument design process. We utilize the clinical experience that will tailor products to better meet patient and surgeon needs. Providing exceptional solutions starts with understanding surgeon customers and the challenges they face. Our team actively listens to and interacts with the surgeon community regarding patient pathologies, complex surgical techniques, and tough clinical challenges. Our team is comprised of an experienced sales force and product development engineers who are available to answer questions and provide technical support as needed.
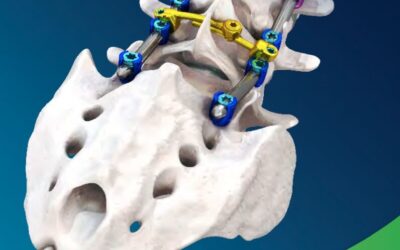
SpineCraft is focused on providing innovative and thoughtful solutions for spine surgery, that help patients lead more active and productive lives. We are focused on research, education, quality and core company values, while still placing tremendous value in our relationships, customer experience and corporate culture. SpineCraft will constantly strive to be the best and will always deliver on top quality over quantity.
Quality statement
Spinecraft’s Quality Policy and Procedures reflect our complete dedication to quality, as well as patient and customer satisfaction. Our comprehensive quality system is designed to achieve high quality standards through strict levels of quality control. This achievement is indicative of the importance placed on meeting patients’ and customers’ needs and is exemplified by the high quality products being manufactured and offered. Management system is in accordance with 21 CFR Part 820 – Medical Devices, Quality System Regulation and GMP. SpineCraft’s Management System also incorporates the requirements of applicable ISO Standards. SpineCraft emphasizes a total quality assurance approach to all aspects of the business. Continuous improvement is the basis for the Quality System. This system provides the structure and oversight to ensure all products are processed, manufactured, controlled and distributed according to the appropriate regulatory requirements and corporate policies and procedures.